Burt Consultancy Portfolio of Developmental Scenarios
The core of the Burt Consultancy portfolio is in early phase development, CNS drug development, and life-cycle management. However, available expertise covers most developmental scenarios, therapeutic areas, and drug classes. The portfolio is a sample of drug development projects that Tal Burt MD and his network colleagues have led. The following Burt Consultancy portfolio examples offer insight into the range of developmental challenges and respective solutions typical of modern drug development.
Also known as Exploratory Investigational New Drug (eIND), and Exploratory Clinical Trials, these approaches to First-in-Human (FIH) studies offer considerable methodological and operational flexibility and advantages over traditional approaches.
The Burt Consultancy portfolio includes several Phase-0/Microdosing programs and developmental scenarios. For more on Phase-0/Microdosing see our recent publication: https://rdcu.be/b61rf
Intra-Target Microdosing (ITM): Portfolio Examples
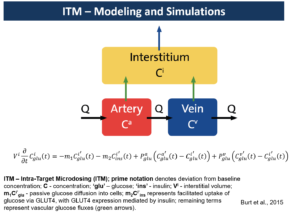
Intra-Target Microdosing (ITM) administers a microdose locally into an area about 1/100th or less of the total body mass. Since microdose is 1/100th or less of the minimal therapeutic-level dose, the exposure in the target will be in the therapeutic range. Such exposure will be short, ranging from seconds to minutes, depending on perfusion parameters. However, it could allow collection of important biomarkers or surrogate endpoints. Such data could be critical to assessment of mechanism of action (MOA).
This can be especially useful if done in patients at the first in human (FIH) study. Regulators have encouraged use of microdosing in patients at the FIH stage (ICH M3 Section 7). The following are examples of developmental scenarios where ITM application would lead to considerable advantages over traditional approaches (from Burt et al. 2016):
localized administration of an NMBD into the radial artery would have effects on hand muscles within seconds to minutes (1,2). Reduction in muscle strength, measured as percent ‘twitch response’, serves as the biomarker associated with drug efficacy. Electrical stimulus is applied to the proximal ulnar nerve to induce a depolarization with the subsequent release of acetylcholine (Ach) at the neuromuscular junction. The resulting contraction of the adductor pollicis muscle is the ‘twitch response’. This response is calibrated as a full twitch (100%). When the NMBD is administered, the reduction of the twitch can be graded (0 - 100%) where 0% represents full neuromuscular blockade. The pharmacodynamic action of the drug (onset, duration of blockade and offset) can then be recorded in terms of % twitch.
This approach represents considerable advantage over current NMBD development process whereby a dose is administered systemically with resultant complete muscle paralysis requiring full ventilatory support. In addition, systemic NMBD studies are conducted under general anesthesia to avoid the extreme anxiety-provoking experience of complete paralysis. The utility of ITM would be the ability to administer NMBD peripherally to a circumscribed target for the purpose of studying its effects while sparing the rest of the body unnecessary exposure and expensive procedures. Furthermore, reversal of NMBD residual muscular block is of clinical importance and could also be studied in a safe and controlled manner using ITM in the early clinical development of NMBDs (3,4). This could prove of particular value in vulnerable populations such as children and elderly (5,6).
An SGLT-2 (Sodium/Glucose Transporter Type 2) inhibitor is administered into the renal artery. Glucose reabsorption into the proximal tubule is thus inhibited and glucose is excreted in the urine (7). The biomarkers would be urine glucose, obtained by catheter in real time, and 18F-FDG excretion and reabsorption (a proxy of glucose excretion and reabsorption), or 11C-labeled drug as measured by dynamic PET-imaging.
Natriuretic peptide (analogue of the naturally occurring Atrial Natriuretic Peptide [ANP] and Brain Natriuretic Peptide [BNP]), a proposed vascular modulator with cardio-protective properties, is administered into the radial artery (8). Vasodilatation and cGMP concentrations serve as physical and chemical biomarkers, respectively, of drug action, and have been shown to take place within minutes of infusion 9. Vasodilatation, conveniently studied in peripherally accessed vessels, is measured using venous occlusion plethysmography. Plasma cGMP concentrations are measured using enzyme immunoassay (9).
A chemotherapeutic agent for hepatocellular carcinoma is administered into the hepatic artery (10). The drug or a biomarker (e.g., FDG) is radiolabelled. Demonstration of binding to tumor tissue through PET-imaging will be proof-of-concept that the chemotherapeutic agent reaches, penetrates and binds to cellular targets (11). Displacement studies could confirm binding to target of interest by administering competitive agonists or antagonists (12). Chemical biomarkers could be obtained in the downstream vein. If done during surgery, blood samples could be more easily obtained, and tissue samples of the tumor could be obtained for histological markers of therapeutic or toxic effects. Since the concentration returning to the systemic circulation becomes a microdose the traditional microdose study where systemic PK and binding to extra-hepatic tissues can be studied as well. Dynamic PET-imaging obtained to show concentrations over time could help study intra-tumour PK, systemic microdose PK, and together with the chemical data could be used to scale results to the whole-body full-pharmacological exposure (13).
The following are Dr. Burt's publications on Phase-0/Microdosing, including Burt Consultancy portfolio projects:
Intra-Target Microdosing (ITM) - A Novel Drug Development Approach: Proof of Concept, Safety, and Feasibility Study in Humans. Clin Transl Sci (2017), 1–20; PMID: 28689370
This is the first human study using the ITM methodology. The study demonstrated the proof-of-concept and feasibility of ITM. After administering insulin microdose into the radial artery the ipsilateral hand received therapeutic level exposure. These exposure allowed detection of biomarkers relevant to insulin action. At the same time, the rest of the body, including the contralateral hand, received microdose exposures. This allowed modeling of the microdose-to-therapeutic-level range of exposures in the same individual.
Intra-Target Microdosing (ITM): A Novel Drug Development Approach Aimed at Enabling Safer and Earlier Translation of Biological Insights into Human Testing. Clinical and Translational Science. 10, 1-14, (2017). PMID: 28419765
This is the ITM concept manuscript describing the range of application and modeling support.
"Phase-0/microdosing studies using PET, AMS, and LC-MS/MS: A range of study methodologies and conduct considerations. Accelerating development of novel pharmaceuticals through safe testing in humans - A practical guide." Expert Opin Drug Deliv. Aug 26. [Epub ahead of print]. PMID: 27564533
This article is a practical guide into Phase-0 approaches. It emphasizes the importance of initiating planning in advance (1.5.-2 years prior to IND) in order to make full use of the potential of these approaches. It also includes the first economic analysis on the impact of microdosing on the patent life of back-up compounds.
"Microdosing and Other Phase-0 Clinical Trials: Facilitating Translation in Drug Development." Clin Transl Sci. 9(2): 74-88. PMID: 26918865
This article is a review of the applications and methodologies available with Phase-0/Microdosing approaches.
Intra-Arterial Microdosing (IAM): A Novel Drug Development Approach, Proof-of-Concept PET Study in Rats. J Nucl Med. Aug 27. 56(11):1793-9. PMID: 26315828
This is the first ITM POC study in animals. Insulin was administered locally at microdose to generate therapeutic level exposures.
Microdosing and drug development: past, present and future. Expert Opin Drug Metab Toxicol 9, 817-34. PMID: 23550938
This is a meta-analysis of 35 studies that included exposures to microdose and therapeutic-level doses. It demonstrates that nearly 80% of drugs administered orally at microdose will extrapolate linearly to therapeutic level exposures. It also demonstrates that if administered IV 100% of the drugs studies extrapolate linearly to therapeutic level exposures.
CNS Drug Development Consultancy
The Burt Consultancy portfolio includes a range of CNS clinical development projects. These include development of drugs and devices for psychiatric and neurological disorders (e.g., mood and anxiety disorders, neurodegenerative conditions, pain).
Examples of CNS developmental scenarios from Dr. Burt's career. These Burt Consultancy portfolio projects highlight unique challenges and their respective solutions:
A lead candidate of an otherwise promising first-in-class anti-depression and anti-anxiety drug was doomed for termination. The reason was that 2 of the preclinical species returned with thyroid black as coal. This is a rare condition and the clinical team, not able to obtain from the preclinical group satisfactory explanations for the finding, decided to terminate the drug. Dr. Burt initiated an investigation and reached out and convened a team of stakeholders all with expertise relevant to the findings. These included endocrinologists, experts in the drug class, liver metabolism experts (since that was the suspected site of production of the pigment), and regulators. The conclusion was that the modification that added the pigmentation led to accumulation in the thyroid. It was further concluded that the product of the modification was chemically inert and pathologically benign. A consultation with the FDA revealed that the agency has had experience with such findings in the past. In one such case (quetiapine) the dossier was obtained through freedom of information (FOI). It confirmed the agency's lack of concern with the 'black thyroid' findings. The candidate drug proceeded to clinical development.
Kamijima, K., Kuboki, T., Kumano, H., Burt, T., Cohen, G., Arano, I. & Hamasaki, T. (2005). A placebo-controlled, randomized withdrawal study of sertraline for panic disorder in Japan. Int Clin Psychopharmacol 20, 265-73. PMID: 16096517
Kamijima, K., Burt, T., Cohen, G., Arano, I. & Hamasaki, T. (2006). A placebo-controlled, randomized withdrawal study of sertraline for major depressive disorder in Japan. Int Clin Psychopharmacol 21, 1-9. PMID: 16317311
Sertraline was slated for re-development in Japan after the failure of an initial bridging strategy. The Japanese authorities raised ethical concerns due to availability of multiple antidepressants in the market. The concern was that patients in the required placebo group will be deprived known effective treatments. Dr. Burt led a team that oversaw the development program with a randomized-withdrawal design. Under the design all patients were initially given sertraline. Those that responded were randomized to sertraline or placebo. The design ensured that immediately upon re-emergence of depressive symptoms patients were discontinued from the study. These withdrawal depression symptoms (the primary outcome) were then managed with approved antidepressants. A similar study was done for panic disorder indication. Both studies were successful leading to approval for depression and panic disorder indications.
This was a large regulatory Phase-3 placebo-controlled study of an antidepressant for the treatment of major depression. The study had a pre-determined interim analysis included in study design. The interim analysis showed lack of separation between drug and placebo. In addition, the analysis showed discrepancies between the rating scales for depression. For example, the Hamilton Rating Scale for Depression (HRSD) had incongruent disease severity ratings when compared with the Clinical Global Depression (CGI) scale. The team decided to re-train the raters including the investigators. The team also visited the sites to observe research subject management. It identified several practices that had high potential for inflation of placebo response. An intensive and targeted training program was initiated. Inter-rater reliability was tested with multiple case studies. The intervention was effective. The second half of the study significant separation of drug from placebo. The separation was large enough to compensate for the lack of separation in the first half of the study. The study was successful and led to approval.
The following are additional examples of Burt Consultancy portfolio of CNS drug development projects:
Thyroid Autoimmune Antibodies and Major Depressive Disorder in Women. Ann Acad Med Singapore 44(8), 284-9. PMID: 26477960
Antidepressant Regulatory Warnings, Prescription Patterns, Suicidality and Other Aggressive Behaviors in Major Depressive Disorder and Anxiety Disorders. Psychiatr Q. Aug 25 PMID: 26303613
Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharmacol 27, 28-34. PMID: 17224709
Gender differences in clinical presentation and response to sertraline treatment of generalized anxiety disorder. Hum Psychopharmacol 20, 3-13. PMID: 15551351
Randomized trial of sertraline versus venlafaxine XR in major depression: efficacy and discontinuation symptoms. J Clin Psychiatry 66, 1312-20. PMID: 16259546
Optimal length of antidepressant trials in late-life depression. J Clin Psychopharmacol 25, S34-7. PMID: 16027559
Sertraline in generalized anxiety disorder: efficacy in treating the psychic and somatic anxiety factors. Acta Psychiatr Scand 111, 429-35. PMID: 15877709
Efficacy, safety, and tolerability of sertraline in patients with late-life depression and comorbid medical illness. J Am Geriatr Soc 52, 86-92. PMID: 14687320
A randomized, placebo-controlled study of the efficacy and safety of sertraline in the treatment of the behavioral manifestations of Alzheimer's disease in outpatients treated with donepezil. Int J Geriatr Psychiatry 19, 9-18. PMID: 14716694
Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci 58, M1137-44. PMID: 14684712
Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol 5, 73-103. PMID: 12057034
The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol Behav Neurol 14, 53-62. PMID: 11234909
Forced titration as a confound in an SSRI comparator study of sertaline versus citalopram in the treatment of major depressive disorder. Biol Psychiatry 50, 66-8. PMID: 11478291
Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry 47, 287-95. PMID: 10686263
Learning and memory in bipolar and unipolar major depression: effects of aging. Neuropsychiatry Neuropsychol Behav Neurol 13, 246-53. PMID: 11186160
Proof-of-Concept (POC) Clinical Development Consultancy
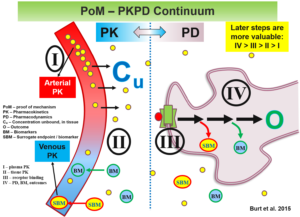
The Burt Consultancy portfolio is a testament to the challenging complexity and multidisciplinary nature of modern drug development. This is especially true at the proof-of-concept (POC) stages (Phase-0/1/2). At these stages, the studies are small, short, and hence often under-powered. To be successful, developmental programs need to incorporate cutting-edge input from a range of disciplines including regulatory sciences, ethics, economics, imaging, machine learning.
This is in addition to obvious disciplines such as clinical pharmacology and statistics. Even the latter ones nowadays include innovations and sub-specializations such as population PK, PBPK modeling and simulations, Bayesian statistics and adaptive designs. These advances need to be considered to maximize signal detection and ensure competitive advantage. To further complicate matters, each test article comes with its unique characteristics, associated tissue and chemical targets, and relevant therapeutic areas. The combination of these factors is unlikely to have a legacy or precedent to readily replicate or learn from.
The under-powered nature of POC studies can be partially addressed using signal-enhancing approaches. For example, the use of biomarkers as surrogate endpoints is particularly valuable for providing precious first-in-human (FIH), and first-in-patient mechanism-of-action (MOA) data. Such use of ‘omics’ approaches (e.g., pharmacogenomics, pharmacometabolomics, pharmacoproteomics), imaging modalities, and advanced analytical methodologies, may allow sufficient variance reduction to compensate for the under-powered nature of POC studies permit arrival at informed developmental decisions.
The following are examples from Dr. Burt's publications and the Burt Consultancy portfolio that emphasize the above points:
- Burt, T., Button, K. S., Thom, H. H. Z., Noveck, R. J., Munafò, M. R. The Burden of the 'False-Negatives' in Clinical Development: Analyses of Current and Alternative Scenarios and Corrective Measures. Clin Transl Sci (2017), 1–10; PMID: 28675646
- Nandal, S. & Burt, T. Integrating Pharmacoproteomics into Early-Phase Clinical Development: State-of-the-Art, Challenges, and Recommendations. International journal of molecular sciences 18, (2017). PMID: 28218733
- Burt T, Dhillon S. (2016). "Pharmacometabolomics in Early-Phase Clinical Development." Clin Transl Sci. Apr 29. PMID: 27127917
- Burt, T. & Dhillon, S. (2013). Pharmacogenomics in early-phase clinical development. Pharmacogenomics 14, 1085-97. PMID: 23837482
- Burt, T., Dhillon, S., Sharma, P., Khan, D., Mv, D., Alam, S., Jain, S., Alapati, B., Mittal, S. & Singh, P. (2013). PARTAKE survey of public knowledge and perceptions of clinical research in India. PLoS One 8, e68666. PMID: 23874712
- Choi YJ, Beck SH, Kang WY, Yoo S, Kim SY, Lee JS, Burt T. and Kim TW. (2016). "Knowledge and Perception about Clinical Research Shapes Behavior: Face to Face Survey in Korean General Public." J Korean Med Sci 31(5): 674-681. PMID: 27134486
References
- Foldes, F. F. et al. The Neuromuscular Effects of ORG9426 in Patients Receiving Balanced Anesthesia. Anesthesiology 75, 191-196 (1991).
- Greenberg, S. B. & Vender, J. The use of neuromuscular blocking agents in the ICU: where are we now? Crit Care Med 41, 1332-1344, doi:10.1097/CCM.0b013e31828ce07c (2013).
- Murphy, G. S. et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesthesia and analgesia 117, 133-141, doi:10.1213/ANE.0b013e3182742e75 (2013).
- Schaller, S. J., Fink, H., Ulm, K. & Blobner, M. Sugammadex and neostigmine dose-finding study for reversal of shallow residual neuromuscular block. Anesthesiology 113, 1054-1060, doi:10.1097/ALN.0b013e3181f4182a (2010).
- McDonagh, D. L. et al. Efficacy, safety, and pharmacokinetics of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in elderly patients. Anesthesiology 114, 318-329, doi:10.1097/ALN.0b013e3182065c36 (2011).
- Yang, C. I. et al. The effect of cisatracurium and rocuronium on lung function in anesthetized children. Anesthesia and analgesia 117, 1393-1400, doi:10.1213/ANE.0b013e3182a6d191 (2013).
- Vasilakou, D. et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Annals of internal medicine 159, 262-274, doi:10.7326/0003-4819-159-4-201308200-00007 (2013).
- Venugopal, J. Pharmacological modulation of the natriuretic peptide system. Expert opinion on therapeutic patents 13, 1389-1409, doi:doi:10.1517/13543776.13.9.1389 (2003).
- Minami, H., Yasu, T., Tagawa, T., Yamakawa, K. & Ueda, S. Slower onset of vasodilating action of brain natriuretic peptide (BNP) compared to atrial natriuretic peptide (ANP) in human forearm resistant vessels. Eur J Clin Pharmacol 64, 859-862, doi:10.1007/s00228-008-0516-4 (2008).
- Yokoyama, J., Ito, S., Ohba, S., Fujimaki, M. & Ikeda, K. A novel approach to translymphatic chemotherapy targeting sentinel lymph nodes of patients with oral cancer using intra-arterial chemotherapy - preliminary study. Head & neck oncology 3, 42, doi:10.1186/1758-3284-3-42 (2011).
- Song, M. J. et al. Predictive value of (1)(8)F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol 18, 3215-3222, doi:10.3748/wjg.v18.i25.3215 (2012).
- Bench, C. J. et al. The time course of binding to striatal dopamine D2 receptors by the neuroleptic ziprasidone (CP-88,059-01) determined by positron emission tomography. Psychopharmacology 124, 141-147 (1996).
- Heuveling, D. A. et al. Phase 0 Microdosing PET Study Using the Human Mini Antibody F16SIP in Head and Neck Cancer Patients. J Nucl Med, doi:10.2967/jnumed.112.111310 (2013).